IMMOBILIZED CELL TECHNOLOGY (ICT) IN BEER FERMENTATION – A POSSIBILITY FOR ENVIRONMENTALLY SUSTAINABLE AND COST-EFFECTIVE PROCESS
Viktor A. Nedovic1, Ida Leskosek-Cukalovic1, Gordana Vunjak-Novakovic2
1Institute of Food Technology and Biochemistry, Faculty
of Agriculture, University of Belgrade, Nemanjina 6, P.O. Box 127, 11081
Belgrade-Zemun
2Department of Chemical Engineering, MIT, Cambridge,
USA
INTRODUCTION
Whole cell immobilization has been defined as "the physical confinement
or localization of intact cells to a certain defined region of space with
preservation of some desired catalytic activity" (Karel et al., 1985).
Many microorganisms own the capability to adhere to different kinds of
surfaces in nature on which way are in close proximity to nutrients and
easy realize a food supply. Therefore, we can say that these biological
systems in their natural state are immobilizad.
However, many biotechnological processes need to be carried out using
immobilization of biocatalysts. Thereby several different techniques and
support materials have been proposed for the cell immobilization in vitro.
Figure 1 illustrates basic methods for immobilization. These techniques
can be divided into four major groups based on the physical mechanism causing
immobilization: physical entrapment within a porous matrix, attachment
or adsorption to a pre-formed carrier, self agregation by floculation (natural)
or crosslinking agents (artificially induced) and cell contained behind
barrier (Pilkington et al., 1998).
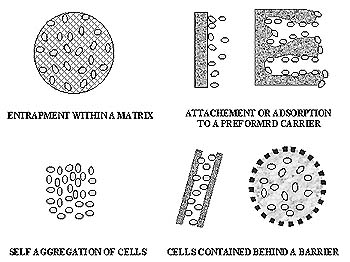
Figure 1 Principal methods of cell immobilization
All of these methods have the same purpose: to retain high cell concentrations
within "a certain defined region of space" such as a bioreactor giving
increased volumetric productivity of a system.
During the last thirty years a numerous different kinds of cell-supporting
materials for immobilization have been developed, such as polymeric matrices
(alginate, agar, gelatin, k-karrageenan, chitosan, pectin, polyacrylamide,
epoxy resin, silica sol), and porous and non-porous preformed support materials
(wood chips, stainless steel, volcanic rock, cotton cloth, porous glass
particles, DEAE cellulose, porous silica, porous ceramics, diatomaceous
earth). The choice of cell-supporting material for any specific application
is related to the following points:
- bioreactor configuration
- high carrier activity
- availability of the carrier in commercial qualities
- low cost of immobilization
- immobilization easy and controllable
- ease of operation scale-up
- excellent mechanical strength during long-time operation
- physiological and environmental safety of the materials used
- low affinity to contaminations
Entrapment in insoluble Ca alginate gel is recognized as a rapid, nontoxic,
inexpensive, versalite and the most often used method for immobilization
of cells (more than 80% of cell immobilization processes are still carried
out using alginate). Alginates make a family of naturally occuring unbranched
binary copolymers, which are formed of D-mannuronic and L-guluronic acids
linked by 1,4-glycosidic bonds. The monomers are arranged in a pattern
of blocks along the chain, with homopolymeric regions (termed M and G blocks)
interspersed with regions of alternating structure-MG blocks (Thu et al.,
1996, 1996). Alginates are differing among themselves by the monomeric
composition and the block structure, and these properties are connected
with the type of algae and the tissue they are isolated from. In the presence
of monovalent cations alginates are forming water solubile salts, but with
polyvalent cations, such as Ca2+, Ba2+ and Sr2+,
they are forming inert polymer network by binding the polyvalent cation
(ussualy Ca2+) to guluronic acid units. This property of alginates
is used for entrapping of living cells in the alginate matrix. The procedure
consists of mixing of cell suspension with Na-alginate solution and dripping
of it into a solution containing divalent cations. This is causing instantaneous
gel spheres formation and cell entrapment into a three dimensional lattice
structure of ionically cross-linked alginate. Since the conditions for
the cells during this procedure are very mild, cells are remaining viable
and catalytic active for a long period of time. There are many examples
of applications of these systems in different fields of industry, medicine
and agriculture.
Immobilization offers many potential advantages over free cell systems,
such as:
- Higher cell densities and cell loads
- Increased volumetric productivity
- Shorter overall reaction times
- Smaller fermenter sizes which may lower capital costs
- The reuse of the same biocatalysts for prolonged period of time due to constant cell regeneration
- A continuous process which may be performed beyond the nominal washout rate
- Improved substrate utilization
- Reduced risk for microbial contamination
- Process design simplified
- Constant product quality
- Improved tolerance or protection of cells from substrate and end-product inhibition.
In technology of food and beverages production, immobilized cell systems are currently used for the production of specific metabolites (Norton, S., and Vuillemard, J.-C., 1994) such as enzymes, amino acids, alcohols, aroma compounds, polysaccharides, pigments, and fermented products such as beer (Masschellein et al., 1994, Nedovic et al., 1996; Kronlof et al., 1996), wine (Divies et al., 1994), cider (Simon et al., 1996; Durieux et al., 1996, Nedovic et al., 1998), vinegar (Mori, 1993), sake (Nunokawa and Hirotsune, 1993), soy sauce (Motai et al., 1993), meat (Norton, S., and Vuillemard, J.-C., 1994), or dairy products (Lacroix et al., 1996; Sodini et al., 1996).
BEER PRODUCTION WITH IMMOBILIZED YEAST CELLS
The production of fermented beverages such as lager-type beer require
a fermentation time of 6-7 days as well as large scale fermentation and
storage capacities. Present trends in immobilized yeast cell technology
nowadays provide the brewing industry with a method of reducing processing
time without sacrificing product quality. By increasing the yeast cell
concentration in the bioreactor vessel, it is possible to accomplish a
faster fermentation and consequently much higher volumetric bioreactor
productivity of beer with significant reduction in fermentation costs.
The earliest applications of immobilized cells to brewing were described
by works of some authors during '70s (Narzzis and Hellich, 1971, Baker
and Kirsop, 1973, White and Portno, 1978). The early packed-bed reactors
consisted of mixture of brewer's yeast and diatomaceous earth forming a
porous biomass bed (Narzzis and Hellich, 1971, Baker and Kirsop, 1973).
Rapidly and efficiently produced beer had the flavour which did not compare
well with that of a similar beer made by conventional fermentation. The
resultant beer had a high concentration of diacetyl, and low of higher
alcohols and esters. Also, the amino acid uptake have been frequently reported
to be low (Pardonova et al., 1982). This and many other studies have found
that amino acid metabolism is obviously critical to beer quality because
the amino acid metabolism is closely linked to production of flavour compounds
such as the vicinal diketones, higher alcohols, organic acids, and sulphur
compounds (Masschelein et al., 1994). The main reason for this unbalanced
metabolic behaviour was altered growth pattern of immobilized cells caused
by mass transfer limitations. In terms of improving amino acid utilisation
as well as beer quality and fermentation efficiency, bulk mixing of the
liquid phase and consequently improved mass transfer is likely to play
an important role.
Thereby in beer brewing with immobilized yeast cells reactor design
has a leading role not only in system selection but also in system efficiency.
Since insufficient mass transfer, i.e. transport of nutrients to yeast
cells, and removal of fermentation by-products from the immobilization
matrix, has been identified as the main factor that causes the unbalanced
flavour profile in beer produced using immobilized yeast packed bed reactors
it was important that extensive care should be taken in assessing and improving
mass transfer in immobilized cell bioreactors. Significant improvement
has been achieved by switching from packed bed to fluidized bed reactors.
Liquid fluidized beds are suitable for support particles that are significantly
more dense than the fermentation medium, because a less dense particle
would be carried upward in these configurations. These systems give much
improved contact between the immobilized yeast and the substrate, which
undoubtedly increases the productivity and permits shorter residence times.
However, mixing may also cause abrasion of the support material, thus affecting
the efficiency of the system
Latest developments in brewing processes with immobilized cells clearly
indicate that by the use of different reactor and system designs, such
as two-stage multi-channel loop reactor system (Andries et al., 1996),
fluidized bed reactor with wort recirculation (Shindo et al., 1994), multi-stage
reactor system (Inoue, 1995) and gas-lift bioreactor system (Nedovic et
al., 1993; Nedovic et al., 1996; Mensour et al., 1996), and a wide range
of beads nature, much better results in a quality of produced beer are
accomplished, than by the use of classic packed bed reactors.
GAS-LIFT SYSTEM
Gas circulating bed reactors can combine the advantages of fluidized
beds, such as high loading of solids and good mass transfer, with the efficient
mixing and controlled liquid flow which are inherent for gas-lift reactors
(Vunjak-Novakovic et al., 1992). The absence of mechanical agitation creates
a relatively low shear environment, which make these reactors ideally suitable
for the application of shear sensitive cells and solid matrix. The solid
phase in external or internal loop bioreactors can be fluidized and kept
in suspension due to circulation of liquid phase and gas bubbles. Low dense
alginate and carrageenan gel particles are typically used in three-phase
gas-lift reactors as carriers of yeast cells in beer fermentations (Nedovic
et al., 1996; Mensour et al., 1996). Since Ca alginate is hydrogel, its
density is very close to that of water and consequently near that of wort.
Mixing requirements for these particles will therefore be relatively low
as compared to heavier matrices such as glass or ceramic beads. The other
important characteristics of gas-lift bioreactors for biochemical processes’
applications are simple construction, low risk of contamination, easy adjustment
and control of the operational parameters and simple capacity enlargement
(Nedovic et al., 1997).
With an interest to produce beer of acceptable quality using immobilized
yeast cells we have designed and studied during the several last years
a three-phase internal loop gas-lift bioreactor used in conjunction with
yeast cells (Saccharomyces cerevisiae) entrapped in small sized Ca-alginate
beds.
The experimental unit was the internal loop gas-lift bioreactor
with a conical bottom, schematically shown in Figure 2. This reactor consisted
of glass column, gas distributor and glass internal draft tube. The fermenting
wort in the reactor was divided into two distinct zones where only one
of them (internal zone) was spargged by nitrogen. Gas phase was introduced
through a central fritted glass sparger at the conical bottom. The different
gas hold-up in the gassed and ungassed zones resulted in different bulk
densities of the fermenting wort in these regions which caused circulation
and mixing of the wort and beads in the reactor by gas-lift action. The
part of the reactor containing the gas-wort-beads upflow is the riser and
the region containing wort and beads downflow is known as the downcomer.
Solid phase was fluidized alginate particles with immobilized yeast cells,
produced by a droplet generator (FTM, Belgrade), and gellated in calcium
chloride (60 min).
Series of fermentations of hopped wort were carried out in this
bioreactor with the same immobilized cell load.
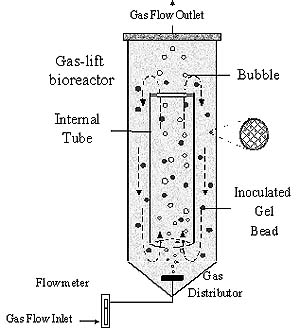
Figure 2 Schematic presentation of the experimental gas-lift biorector
Main results:
· Primary beer fermentation, which takes 6-7 days in traditional
brewing, was drastically shortened to only about 16 hours.
· Obtained results also showed insignificant differences in
chemical composition and sensory characteristics between beers obtained
with immobilized cells and conventionally produced beers. The concentrations
of higher alcohols and esters were at the same level as those obtained
by the fermentation with suspended cell.
· During the 3-5 months period the same immobilized cell load
of unchanged viability and activity was utilized.
· Within the range of superficial gas velocity between 0.03
and 0.2 cm/s fermentation efficiency showed increasing trend and after
that further increasing in superficial gas velocity little bit changed
the fermentation efficiency.
· Superfficial gas velocity was also the main parameter for
amino-acid nitrogen uptake during the fermentation. The increase of superficial
gas velocity leads to the increase of amino acid consumption. The final
amino-acid concentration was similar to those obtained during the conventional
brewing, and even lower than that.
· By comparing the maximum obtained value of fermentation rate
in the bioreactor with values obtained in packed bed and fluidized bed
reactors one can conclude that internal loop gas-lift bioreactor is much
more efficient among similar systems. It can be explained by liquid mixing
characteristics of the bioreactor, i.e. excellent liquid-solid and gas-liquid
mass transfer within the system, with a balanced flavour profile of finished
product, as a consequence.
INDUSTRIAL SCALE APPLICATION AND ECONOMICAL AND ENVIRONMENTAL ASPECTS
Above all previously mentioned facts there are also several very
significant economical and environmental reasons confirmed through practice
that clearly indicate advantages of the immobilized yeast cell technology
introduction in the industrial beer processing.
- Proposed immobilized cell technology is very simple for introduction
in beer processing. Compact continuously operated processes will increase
flexibility in production planning, and production losses will be smaller.
- Initial savings could be gained by reduction in investment costs
(reduced number and size of the fermentors as well as minimal space requirements),
while further savings could be gained in reduced operating costs.
- There is great variety of different yeast carriers. Some of them
are easily reusable and can have a lifetime of more than 10 years.
- The utilization of yeast carriers leads furthermore to the reduction
of kieselguhr consumption in filtration, which makes handling easier and
cheaper.
- Application of immobilized cell technology will decrease environmental
load, because immobilized yeast reduce waste yeast load, as new biomass
is not produced in conventional quantities. Furthermore, reduced product
losses, reduced water consumption for planning purposes and smaller cleaning
chemicals consumption also have positive environmental effects.
LITERATURE
1. Andries, M., van Beveren, P.C., Goffin, O., Masschelein, C.A. Eur.
Brew. Conv., Monograph XXIV, 134-144, 1996
2. Baker, D.A., and Kirsop, B.H., J. Inst. Brew. 79, 487-494, 1973
3. Divies, C., Cashon, R., Cavin, J.-F., and Prevost, H., Critical
Reviews in Biotechnology 14(2), 135-153, 1994
4. Durieux, A., Garre, V., Mukamana, J., Jourdain, J.-M., Silva, D.,
Plaisant, A.-M., Defroyennes, J.-P., Foroni, G., and Simon, J.-P., Progress
in Biotechnology 11, Immobilized Cells: Basics and Applications, Wijffels,
R.H., Buitelaar, R.M., Bucke, C., Tramper, J., eds., Elsevier Science B.V.,
679-687, 1996
5. Inoue, T., Eur. Brew. Conv., Proceedings of the 25th Congress, Brussels,
25-36, 1995
6. Karel, S.F., Libicki, S.B., and Robertson, C.R., Chemical Engineering
Science 40, 1321-1354, 1985
7. Kronlof, J., Linko, M., and Pajunen, E., EBC Monograph XXIV, Symposium
on Immobilized Yeast Applications in the Brewing Industry, ESPOO, 118-124,
1996
8. Lacroix, C., Sodini, I., and Corrieu, G., Progress in Biotechnology
11, Immobilized Cells: Basics and Applications, Wijffels, R.H., Buitelaar,
R.M., Bucke, C., Tramper, J., eds., Elsevier Science B.V., 600-608, 1996
9. Masschellein, C.A., Ryder, D.S., and Simon, J.-P., Critical Reviews
in Biotechnology 14(2), 155-177, 1994
10. Mensour, N., Margaaritis, A., Briens, C.L., Pelkington, H., Russell,
I., Eur. Brew. Conv., Monograph XXIV, 125-132, 1996
11. Mori, A., Industrial Application of Immobilized Biocatalysts, Tanaka,
A., Tetsuya, T., Kobayashi, T., eds., Marcel Dekker, Inc., 291-315, 1993
12. Motai, H., Hamada, T., Fukushima, Y., Industrial Application of
Immobilized Biocatalysts, Tanaka, A., Tetsuya, T., Kobayashi, T., eds.,
Marcel Dekker, Inc., 315-337, 1993
13. Narzzis, L., and Hellich, P., Brauwelt 111, 1491-1500, 1971
14. Nedovic, V., Obradovic, B., Leskosek-Cukalovic, I., Vunjak-Novakovic,
G., Hem. Ind. 47 (11-12), 168-172, 1993
15. Nedovic, V.A., Leskosek-Cukalovic, I., and Vunjak-Novakovic, G.,
Institute of Brewing, Proceedings of the Twenty Fourth Convention, J. Harvey,
ed., Winetitles, Adelaide, 245, 1996
16. Nedovic,V., Pe{i}, R., Lesko{ek-^ukalovi}, I., Laketi}, D., Vunjak-Novakovi},
G., II European Conference on FLUIDIZATION, M. Olazar and M.J.San Jose,
eds., Univ. Basque Country Press Service, 627-635, 1997
17. Nedovic, V., Durieux, A., van Nedervelde, L., Rosseels, P., Vandegans,
J., Plaisant, A-M., Simon, J-P., Proceedings of the EC Symposium "Yeast
as a Cell Factory", P. Osseweijer and J.P. van Dijken, eds., Vlaardingen,
The Netherlands, 188-190, 1998
18. Norton, S., and Vuillemard, J.-C., Critical Reviews in Biotechnology
14(2), 193-224, 1994
19. Nunokawa, Y., Hirotsune, M., Industrial Application of Immobilized
Biocatalysts, Tanaka, A., Tetsuya, T., Kobayashi, T., eds., Marcel Dekker,
Inc., 235-255, 1993
20. Pardonova, B., Polednikova, M., Sedova, H., Kahler, M., Ludvik,
J., Brauwissenschaft 35, 254-258, 1982
21. Pilkington, P.H., Margaritis, A., Mensour, N.A., Russell, I., J.
Inst. Brew. 104, 19-31, 1998
22. Shindo, S., Sahara, S., Watanabe, N., Koshino, S., Institute of
Brewing, Proceedings of the 23rd Convention, Sydney, 109-113, 1994
23. Simon, J.-P., Durieux, A., Pinnel, V., Garre, V., Vandegans, J.,
Rosseels, P., Godan, N., Plaisant, A.-M., Defroyennes, J.-P., and Foroni,
G., Progress in Biotechnology 11, Immobilized Cells: Basics and Applications,
Wijffels, R.H., Buitelaar, R.M., Bucke, C., Tramper, J., eds., Elsevier
Science B.V., 615-622, 1996
24. Sodini, I., Corrieu, G., andLacroix, C., Progress in Biotechnology
11, Immobilized Cells: Basics and Applications, Wijffels, R.H., Buitelaar,
R.M., Bucke, C., Tramper, J., eds., Elsevier Science B.V., 687-695, 1996
25. Thu, B., Smidsrod, O., Skjak-Braek, G. Progress in Biotechnology
11, Immobilized Cells: Basics and Applications, Wijffels, R.H., Buitelaar,
R.M., Bucke, C., Tramper, J., eds., Elsevier Science B.V., 19-31, 1996
26. Vunjak-Novakovic, G., Jovanovic, G., Kundakovic, Lj., Obradovic,
B., Chem. Eng. Sci. 47, 3451-3458, 1992
27. White, F.H., and Portno, A.D., J. Inst. Brew 84, 228-230, 1978
 Viktor
A. Nedovic
Viktor
A. Nedovic
CURRICULUM VITAE
B.Sc. in Food Technology and Biochemistry, Faculty of Agriculture, University of Belgrade.
M.Sc. in Biochemical Engineering, Faculty of Technology, University of Belgrade (Thesis title: Kinetics of Beer Fermentation with Immobilized Yeast Cells in an Internal Loop Air-Lift Bioreactor).
Teaching Assistant for the subject: Brewing and Malting Technology, Institute of Food Technology and Biochemistry, Faculty of Agriculture, University of Belgrade, 1990 to present. Research Associate on several projects in different fields of biotechnology at Dept. of Chem. Engineering and at Inst. of Food Technology and Biochemistry, University of Belgrade, 1990 to present. Visiting Research Associate, Institute Meurice, Dept. of Biotechnology, Campus CERIA, Brussels, Belgium, 1997/1998.
Published and presented over 60 scientific and technical papers in International and Yugoslav Journals and Conferences. Has been invited as a plenary lecturer to two international conferences. Published one monograph. His major research fields are immobilized cell systems, kinetic of biochemical reactions, bioreactor design, beer fermentation, cider fermentation, continuous processes.
Awarded by: The Foundation for young scientists and artists (1990-92), European Commission (EC) - Grant for 1997, Federation of European Microbiological Societies (FEMS) - Fellowship for 1997, EC and FEMS - Grant for 1998, European Congress of Biotechnology - Grant for 1999, Bioencapsulation Research Group - Award for 1999.
A member of Yugoslav Society for Microbiology and Bioencapsulation Research Group (BRG).
Contact address: Institute of Food Technology and Biochemistry, Faculty of Agriculture, University of Belgrade
Nemanjina 6, P.O. Box 127
11081 Belgrade-Zemun, Yugoslavia
Telephone: 381 11 199 711
Fax number: 381 11 193 659 or 199 711
E-mail: vnedovic@eunet.yu
 Ida
Leskosek-Cukalovic
Ida
Leskosek-Cukalovic
SHORT CURRICULUM VITAE
Ida Leskosek-Cukalovi} B.Sc. Engineering - University of Belgrade, Faculty
of Technology and Metallurgy, Belgrade
Ph. D. Biotechnological Science, Faculty of Agriculture, Belgrade
Currently: Associated Professor of Brewing Technology, Faculty of Agriculture,
University of Belgrade
Specialization: Heriot Watt University, Edinburgh, UK
A member of the Institute of Brewing, UK. A member of the Organizing
Committee of the II and III Yugoslav Conference on Food Technology.
A member of the Organizing Committee and invited speaker for the Symposium
on Yugoslav Brewing Industry. A member of the Organizing Committee and
invited speaker for the Symposium on Yugoslav Fermentation Industry. Founder
and head of the Laboratory for Brewing at the University of Belgrade, performing
studies in the field of beer science, alcoholfree beer production, membrane
separation technique, yeast immobilization. Member of several Yugoslav
and international multidisciplinary projects performed by joint efforts
University / National Laboratories / Industry, 1979 - 1999. Published and
presented over 80 scientific and technical papers. Has been teaching undergraduate
and graduate level courses in Brewing Science.
Office address: Institute of Food Technology and Biochemistry
Faculty of Agriculture, University of Belgrade
11080 Beograd-Zemun, Nemanjina 6
Phone: (381-11) 615-315/ ext.353
E-mail: i.les@Eunet.YU