Allotropes of carbon
From Wikipedia, the free encyclopedia
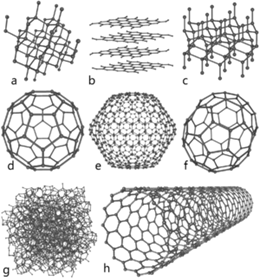
Eight allotropes of carbon: a) Diamond, b) Graphite, c) Lonsdaleite,
d) C60 (Buckminsterfullerene or buckyball),
e) C540, f) C70, g) Amorphous
carbon, and h) single-walled carbon
nanotube or buckytube.
This is
a list of the allotropes of carbon.
Contents
4 Buckminsterfullerenes
(4.1 Buckyballs ; 4.2 Carbon nanotubes ;
4.3 Carbon nanobuds) 8 Lonsdaleite (hexagonal diamond)
|
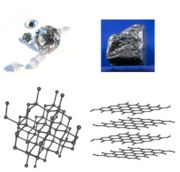
Diamond
Diamond is one of the best known allotropes of
carbon, whose hardness and high dispersion of light make it useful for
industrial applications and jewelry. Diamond is the hardest known natural mineral, which
makes it an excellent abrasive and makes it hold polish and luster extremely
well. No known naturally occurring substance can scratch, let alone cut, a
diamond.
The market for industrial-grade diamonds operates much differently from its
gem-grade counterpart. Industrial diamonds are valued mostly for their hardness
and heat conductivity, making many of the gemological
characteristics of diamond, including clarity and color, mostly irrelevant.
This helps explain why 80% of mined diamonds (equal to about 100 million carats
or 20,000 kg annually), unsuitable for use as gemstones and known as bort, are destined
for industrial use. In addition to mined diamonds, synthetic diamonds found
industrial applications almost immediately after their invention in the 1950s;
another 400 million carats (80,000 kg) of synthetic diamonds are produced
annually for industrial use—nearly four times the mass of natural diamonds
mined over the same period.
The dominant industrial use of diamond is in cutting, drilling (drill
bits), grinding (diamond edged cutters), and polishing. Most uses of diamonds
in these technologies do not require large diamonds; in fact, most diamonds
that are gem-quality can find an industrial use. Diamonds are embedded in drill
tips or saw blades, or ground into a powder for use in grinding and polishing
applications. Specialized applications include use in laboratories as
containment for high pressure experiments (see diamond
anvil), high-performance bearings, and limited use in specialized windows.
With the continuing advances being made in the production of synthetic
diamond, future applications are beginning to become feasible. Garnering much
excitement is the possible use of diamond as a semiconductor
suitable to build microchips from, or the use of diamond as a heat sink
in electronics.
Significant research efforts in Japan, Europe, and the United
States are under way to capitalize on the potential offered by diamond's
unique material properties, combined with increased quality and quantity of
supply starting to become available from synthetic diamond manufacturers.
Each carbon atom in a diamond is covalently bonded to four other carbons in
a tetrahedron. These tetrahedrons together form a 3-dimensional network of
puckered six-membered rings of atoms. This stable network of covalent
bonds and the three dimensional arrangement of bonds is the reason that
diamond is so strong.
Graphite
Graphite (named by Abraham Gottlob Werner in 1789, from the Greek
γράφειν: "to draw/write", for its
use in pencils) is one of the most common allotropes of carbon. Unlike diamond,
graphite is an electrical conductor, and can be used, for instance, as the
material in the electrodes of an electrical arc lamp. Graphite holds the
distinction of being the most stable form of carbon under standard conditions. Therefore, it is used in
thermochemistry as the standard state for defining the heat of formation of carbon
compounds.
Graphite is able to conduct electricity, due to delocalization
of the pi bond
electrons
above and below the planes of the carbon atoms. These electrons are free to
move, so are able to conduct electricity. However, the electricity is only
conducted along the plane of the layers. In diamond all four outer electrons of
each carbon atom are 'localised' between the atoms in covalent bonding. The
movement of electrons is restricted and diamond does not conduct an electric
current. In graphite, each carbon atom uses only 3 of its 4 outer energy level
electrons in covalently bonding to three other carbon atoms in a plane. Each
carbon atom contributes one electron to a delocalised system of electrons that
is also a part of the chemical bonding. The delocalised electrons are free to
move throughout the plane. For this reason, graphite conducts electricity along
the planes of carbon atoms, but does not conduct in a direction at right angles
to the plane.
Graphite powder is used as a dry lubricant.
Although it might be thought that this industrially important property is due
entirely to the loose interlamellar coupling between sheets in
the structure, in fact in a vacuum environment (such as in technologies for use in space),
graphite was found to be a very poor lubricant. This fact led to the discovery
that graphite's lubricity is due to adsorbed air
and water between the layers, unlike other layered dry lubricants such as molybdenum disulfide. Recent studies suggest
that an effect called superlubricity can also account for this effect.
When a large number of crystallographic defects bind these planes together,
graphite loses its lubrication properties and becomes what is known as pyrolytic
carbon, a useful material in blood-contacting implants such as prosthetic heart
valves.
Natural and crystalline graphites are not often used in pure form as
structural materials due to their shear-planes, brittleness and inconsistent
mechanical properties.
In its pure glassy (isotropic) synthetic forms, pyrolytic graphite and carbon
fiber graphite is an extremely strong, heat-resistant (to 3000 °C)
material, used in reentry shields for missile nosecones, solid
rocket engines, high temperature reactors, brake shoes and electric
motor brushes.
Intumescent or expandable graphites are used in fire seals, fitted around
the perimeter of a fire door. During a fire the graphite intumesces (expands
and chars) to resist fire penetration and prevent the spread of fumes. A
typical start expansion temperature (SET) is between 150 and 300 degrees
Celsius.
Density: its specific gravity is 2.3 which makes
it lighter than diamond.
Effect of heat: it is the most stable allotrope of carbon. At a temperature
of 2500 degree Celsius, it can be transformed into diamond. At about 700 degree
Celsius it burns in pure oxygen forming carbon dioxide.
Chemical activity: it is slightly more reactive than diamond. This is
because the reactants are able to penetrate between the hexagonal layers of
carbon atoms in graphite. It is unaffected by ordinary solvents, dilute acids,
or fused alkalis. However, chromic acid oxidises it to carbon dioxide.
Amorphous carbon
Amorphous carbon is the
name used for carbon
that does not have any crystalline structure. As with all glassy
materials, some short-range order can be observed, but there is no long-range
pattern of atomic positions.
While entirely amorphous carbon can be made, natural amorphous carbon (such
as soot) actually contains microscopic crystals of graphite, [1] sometimes diamond [2]. On the macroscopic
scale, amorphous carbon has no definite structure as it conisists of small
irregular cystals, but on the nanomicroscopic scale, we can see it is made of
regularly arranged carbon atoms.
Coal and soot are both
informally called amorphous carbon. However, both are products of pyrolysis
(the process of decomposing a substance by the action of heat), which does not
produce true amorphous carbon under normal conditions. The coal industry
divides coal up into various grades depending on the amount of carbon present
in the sample compared to the amount of impurities. The highest grade, anthracite,
is about 90 percent carbon and 10% other elements. Bituminous
coal is about 75-90 percent carbon, and lignite is the
name for coal that is around 55 percent carbon.
Buckminsterfullerenes
|
Part of a series of articles on |
The buckminsterfullerenes, or usually just fullerenes
for short, were discovered in 1985 by a team of scientists from As of the early twenty-first century, the chemical and
physical properties of fullerenes are still under heavy study, in both pure
and applied research labs. In April 2003, fullerenes were under study for
potential medicinal use — binding specific antibiotics to the structure to
target resistant bacteria and even target certain cancer cells such as
melanoma. |
|
Fullerenes |
|
|
Nanoparticles |
|
|
See also |
|
Buckyballs
Spherical fullerenes are also called buckyballs.
Carbon
nanotubes
Carbon nanotubes, also called buckytubes, are cylindrical carbon molecules
with novel properties that make them potentially useful in a wide variety of
applications (e.g., nano-electronics, optics, materials
applications, etc.). They exhibit extraordinary strength, unique electrical
properties, and are efficient conductors of heat. Inorganic nanotubes have also been synthesized.
A nanotube (also known as a buckytube) is a member of the fullerene
structural family, which also includes buckyballs. Whereas buckyballs are spherical in
shape, a nanotube is cylindrical, with at least one end typically
capped with a hemisphere of the buckyball structure. Their name is derived from
their size, since the diameter of a nanotube is on the order of a few nanometers
(approximately 50,000 times smaller than the width of a human hair), while they
can be up to several centimeters in length. There are two main types of
nanotubes: single-walled nanotubes (SWNTs) and multi-walled
nanotubes (MWNTs).
Carbon
nanobuds
Computer models of stable NanoBud structures
Carbon NanoBuds are a newly discovered
allotrope of carbon
in which fullerene
like "buds" are covalently attached to the outer sidewalls of the carbon
nanotubes. This hybrid material has useful properties of both fullerenes
and carbon nanotubes. In particular, they have been found to be exceptionally
good field emitters.
Aggregated diamond nanorods
Aggregated diamond nanorods, or ADNRs,
are an allotrope
of carbon
believed to be the least compressible material known to humankind, as measured
by its isothermal bulk modulus; aggregated diamond
nanorods have a modulus of 491 gigapascals (GPa), while a conventional diamond has a
modulus of 442 GPa. ADNRs are also 0.3% denser than regular diamond. The ADNR
material is also harder than type IIa diamond and ultrahard fullerite.
Glassy
carbon
Glassy carbon is a class of
non-graphitizing carbon
which is widely used as an electrode material in electrochemistry,
as well as for high temperature crucibles and as a component of some prosthetic
devices. It was first produced by workers at the laboratories of The General Electric Company,
It was first produced by Bernard Redfern in the mid 1950's at the laboratories
of The Carborundum Company,
The preparation of glassy carbon involves subjecting the organic precursors
to a series of heat treatments at temperatures up to 3000oC. Unlike
many non-graphitizing carbons, they are impermeable to gases and are chemically
extremely inert, especially those which have been prepared at very high
temperatures. It has been demonstrated that the rates of oxidation of certain
glassy carbons in oxygen, carbon dioxide or water vapour are lower than those
of any other carbon. They are also highly resistant to attack by acids. Thus,
while normal graphite
is reduced to a powder by a mixture of concentrated sulfuric and nitric acids
at room temperature, glassy carbon is unaffected by such treatment, even after
several months.
Carbon
nanofoam
Carbon nanofoam is the fifth known
allotrope of carbon discovered in 1997 by Andrei
V. Rode and co-workers at the Australian National University in Canberra. It
consists of a low-density cluster-assembly of carbon atoms strung together in a
loose three-dimensional web.
Each cluster is about 6 nanometers wide and consists of about 4000 carbon atoms linked in graphite-like
sheets that are given negative curvature by the inclusion of heptagons among
the regular hexagonal
pattern. This is the opposite of what happens in the case of buckminsterfullerenes, in which carbon sheets
are given positive curvature by the inclusion of pentagons.
The large-scale structure of carbon nanofoam is similar to that of an aerogel, but with
1% of the density of previously produced carbon
aerogels - only a few times the density of air at sea level.
Unlike carbon aerogels, carbon nanofoam is a poor electrical conductor.
Lonsdaleite (hexagonal diamond)
Lonsdaleite is a hexagonal
allotrope of the carbon allotrope diamond, believed to form when meteoric graphite falls
to Earth. The
great heat and stress of the impact transforms the graphite into diamond, but
retains graphite's hexagonal crystal lattice.
Lonsdaleite was first identified from the Canyon Diablo meteorite at Barringer
Crater (also known as Meteor Crater) in Arizona. It was
first discovered in 1967.
Lonsdaleite occurs as microscopic crystals associated with diamond in the
Canyon Diablo meteorite; Kenna meteorite, New Mexico;
and Allan Hills (ALH) 77283, Victoria Land, Antarctica
meteorite. It has also been reported from the Tunguska impact site, Russia.
Linear Acetylenic Carbon (LAC)
Chemists in the
The same polymer was synthesized in early 1960s by group of Soviet chemists
and was called carbyne (Russian:
карбин). It appeared to be a semiconductor
that is very sensitive to light, thus it was suggested to use it in photodiodes
and similar devices.
Carbyne, or polyyne, is also another name for Linear Acetylenic Carbon [1] (LAC) the carbon allotrope that has the chemical structure [2] -(C:::C)n- .Carbon in this modification is
linear with sp orbital hybridisation, and is a polymer with
alternating single and triple bonds. This type of carbyne is of considerable
interest to nanotechnology as its Young's modulus is forty times
that of diamond [3].
Variability
of carbon
Diamond and graphite are two allotropes of carbon:
pure forms of the same element that differ in structure.
The system of carbon allotropes spans an astounding range of extremes,
considering that they are all merely structural formations of the same element.
Between
diamond and graphite:
Diamond crystallizes in the cubic system but graphite crystallizes in
the hexagonal system.
Diamond is hardest mineral known to man (10 on Mohs scale), but graphite is one of
the softest (1 - 2 on Mohs scale).
Diamond is the ultimate abrasive, but graphite is
a very good lubricant.
Diamond is an excellent electrical insulator, but
graphite is a conductor of electricity.
Diamond is an excellent thermal conductor, but
some forms of graphite are used for thermal insulation (i.e. heatshields and
firebreaks)
Other
possible forms
Chaoite is a mineral believed to have been formed in meteorite impacts. It has
been described as slightly harder than graphite with a reflection colour of
grey to white. However, the existence of carbyne phases is disputed – see the
entry on chaoite
for details.
Metallic carbon: Theoretical studies have shown that carbon
(diamond) when brought at enormous pressure, there are
regions in the phase diagram where it's metallic.[4] It seems that it can also become superconducting at very
low temperatures (4 kelvins).[5]
Hexagonite: in theory, instead of having the 6-arom rings of
graphite, one sp carbon atom could be inserted between each of the 6 sp2
atoms.[6]
Prismane
C8 is another possible metastable form.